As a curious explorer navigating the vast expanse of chemistry, I’ve always been fascinated by the intricate dance of electrons whirling around atomic nuclei. In this realm of quantum madness, phosphorus, the 15th element on the periodic table, stands out as a captivating subject. Join me as we unravel the mysteries surrounding the enigmatic electron shells of phosphorus and delve into the heart of its atomic structure.
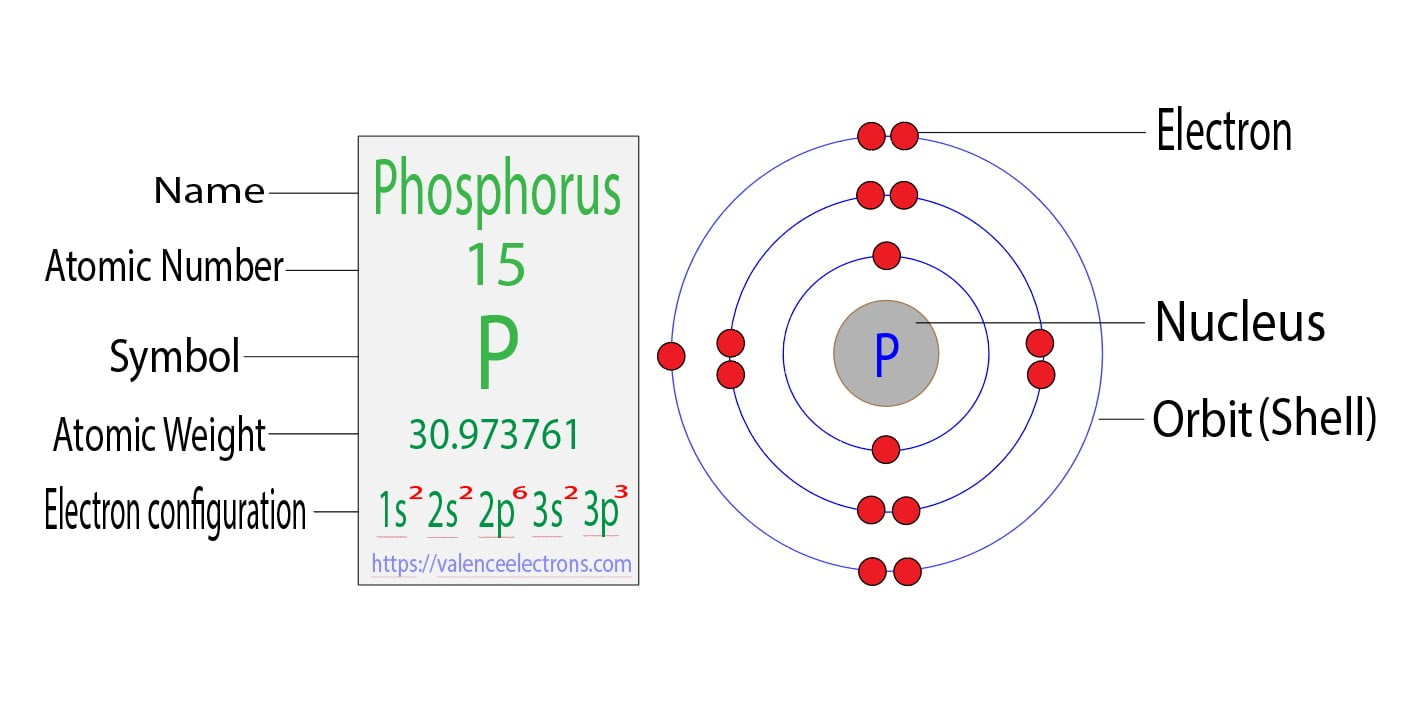
Image: valenceelectrons.com
The Electronic Tapestry of Phosphorus: Unveiling Its Layers
Every atom, including phosphorus, holds a nucleus at its core, a densely packed haven of protons and neutrons. Surrounding this nucleus, electrons occupy designated energy levels, like celestial dancers in an ethereal ballet. These energy levels are organized into concentric electron shells, each possessing a distinct capacity for holding electrons.
Phosphorus, in its quest for electronic harmony, arranges its electrons within three distinct shells. The first shell, closest to the nucleus, dances with a maximum of 2 electrons. The second shell, venturing out further, allows up to 8 electrons to twirl around it. The third and outermost shell of phosphorus accommodates a maximum of 5 electrons, completing its electronic ensemble.
Unveiling the Valence Shell: Phosphorus’s Gateway to Reactivity
Amongst these electron shells, the outermost shell holds a special significance. Known as the valence shell, it dictates the character and reactivity of an element. In the case of phosphorus, its third electron shell, housing 5 electrons, acts as its valence shell.
The valence electrons, the most dynamic and versatile members of the atomic entourage, participate in chemical reactions, granting phosphorus its ability to bond with other elements and engage in a myriad of fascinating chemical processes.
Latest Frontiers in Phosphorus Chemistry: Beyond Electron Shells
The realm of phosphorus chemistry continues to captivate scientists, as they push the boundaries of our knowledge. Researchers are now delving into the depths of molecular phosphorus, exploring the intricacies of its bonds and interactions within complex molecules. These pursuits hold promise for advancements in fields such as materials science, catalysis, and biotechnology.
Social media platforms, forums, and conferences serve as vibrant exchange points for the latest discoveries in phosphorus chemistry. By engaging in these discussions, we as researchers can contribute our insights and stay abreast of the rapidly evolving landscape of this captivating element.
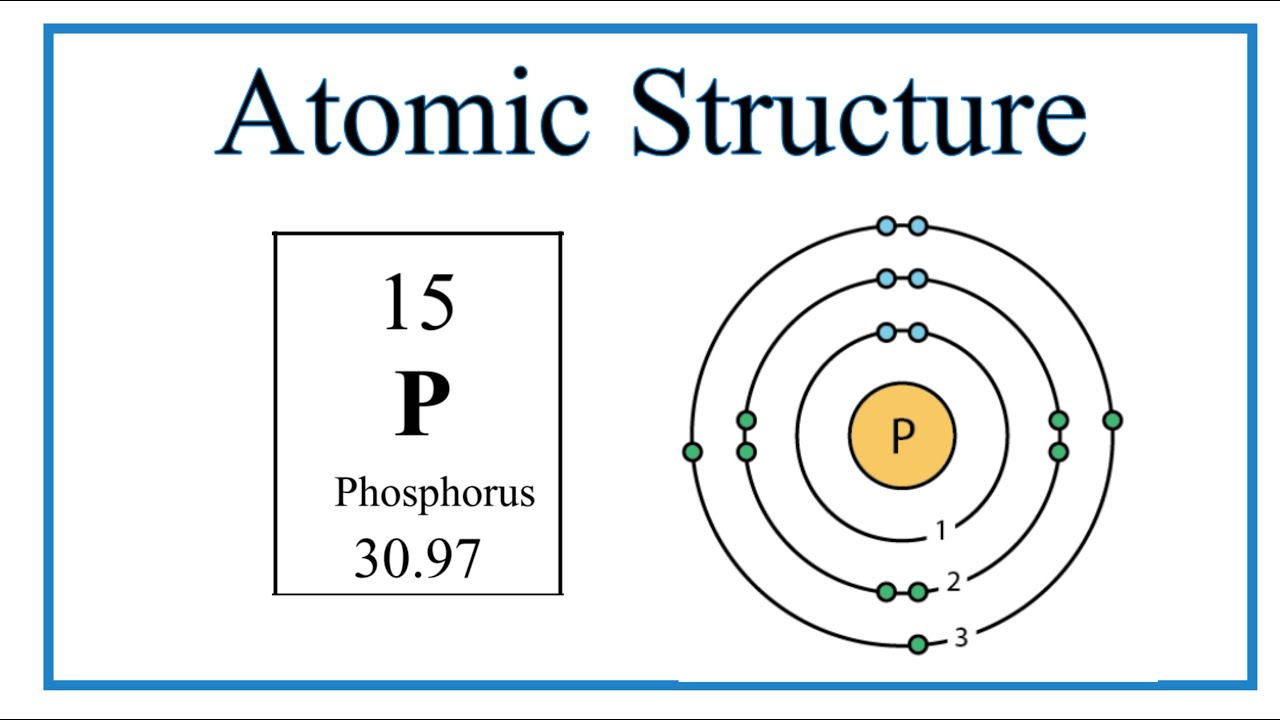
Image: zelengarden.ru
Tips for Navigating the Electronic Realm of Phosphorus
As you explore the fascinating world of electron shells and phosphorus chemistry, keep these tips in mind:
- Use reliable sources: Always consult credible scientific literature and textbooks for accurate information.
- Question and analyze: Don’t just accept facts passively. Challenge information, seek evidence, and engage in critical thinking.
- Visualize concepts: Utilize diagrams, simulations, and animations to enhance your understanding of abstract concepts.
- Seek expert guidance: Don’t hesitate to reach out to professors, researchers, or online forums for clarification and guidance.
- Practice makes perfect: Engage in practice problems and thought exercises to reinforce your comprehension.
Unveiling the Secrets: Phosphorus Unveiled
Q: How many electrons does phosphorus have in its valence shell?
A: Phosphorus has 5 electrons in its valence shell, located in its third and outermost energy level.
Q: What is the electron configuration of phosphorus?
A: The electron configuration of phosphorus is 1s2 2s2 2p6 3s2 3p3, indicating the distribution of its 15 electrons across its energy levels.
How Many Electron Shells Does Phosphorus Have
https://youtube.com/watch?v=OewP_NKiFoE
Conclusion: Phosphorus’s Electron Shells Unraveled
Our exploration into the ethereal realm of electron shells has unveiled the intricate dance of electrons that defines phosphorus, the 15th element. With three energy levels, housing a total of 15 electrons, phosphorus exhibits its unique reactivity through its valence electrons. The pursuit of knowledge in phosphorus chemistry continues, expanding our understanding of this fascinating element.
Dear reader, do you share the same fascination for the intriguing world of electron shells and phosphorus chemistry? Are you eager to delve deeper into the depths of atomic structure and chemical reactivity? If so, embark on this captivating quest and let the allure of phosphorus guide you towards a world of scientific wonder.