Picture this: as a young science enthusiast, I recall poring over countless textbooks, my curiosity piqued by the mysterious numbers etched beside elements in the periodic table. What did these perplexing digits represent? Intrigued, I delved into the depths of atomic mysteries, uncovering the fascinating secrets encoded within those enigmatic numbers.
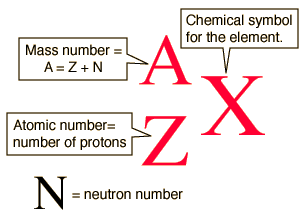
Image: socratic.org
To quench our thirst for knowledge, let us embark on a journey through the realm of isotopes and unravel the profound significance that lies within the numbers that accompany them.
Unraveling the Atomic Nucleolus: A Tale of Mass and Identity
At the heart of every atom resides the atomic nucleus—a densely packed core constituting a tantalizing microcosm of protons and neutrons. Protons, bearing positive electric charges, define an element’s identity, their number assigning each element its unique position on the periodic table. Neutrons, devoid of electric charge, contribute to the atom’s mass, bestowing upon each element its individual atomic weight.
Isotopes emerge as variants of the same element, sharing an identical number of protons but boasting distinct neutron counts. These variations translate into different forms of the same element, each with subtly distinct properties and behaviors. And it is the number imprinted beside the element symbol that holds the key to discerning an isotope’s neutron endowment, a crucial factor in determining its atomic mass, stability, and potential applications.
A Case Study: Carbon and Its Isotopic Cousins
Consider carbon—a ubiquitous element indispensable for life on Earth. Existing in nature as a medley of isotopes, carbon’s most common forms include carbon-12, carbon-13, and carbon-14. The number adjacent to each incarnation signifies the total number of nucleons (protons + neutrons) within the nucleus.
Carbon-12, with 6 protons and 6 neutrons, reigns as the lightest and most abundant carbon isotope, constituting the cornerstone of most organic molecules. Carbon-13, with 6 protons and 7 neutrons, exhibits a subtly altered mass, while carbon-14, featuring 6 protons and 8 neutrons, emerges as a radioactive isotope with profound implications in dating ancient artifacts and fossils.
Navigating the Isotope Landscape: Stable and Radioactive Havens
The world of isotopes encompasses two distinct categories: stable and radioactive. Stable isotopes, such as carbon-12, maintain an unwavering balance of nuclear forces, enduring indefinitely without undergoing radioactive decay. These unwavering isotopes form the foundation of the natural world, comprising the vast majority of elemental forms we encounter.
In contrast, radioactive isotopes, like carbon-14, possess an unstable nuclear composition. Over time, these isotopes undergo spontaneous decay, emitting particles or energy to achieve a more stable configuration. This decay serves as the driving force behind nuclear power, medical imaging, and a myriad of other transformative technologies.

Image: www.coursehero.com
Applications Beyond the Laboratory: Unveiling the Practicality of Isotopes
The realm of isotopes extends far beyond the confines of scientific laboratories, permeating our daily lives in countless ways:
- Industry and Manufacturing: Stable isotopes find applications in metallurgy, tracing the flow of materials through industrial processes and ensuring quality control.
- Medical Diagnostics: Radioactive isotopes empower medical imaging techniques, allowing physicians to visualize and diagnose a wide range of ailments.
- Environmental Science: Isotopic analysis enables scientists to probe environmental processes, uncovering insights into climate change, water cycles, and pollution tracing.
- Archaeology and Paleontology: Radioactive isotopes like carbon-14 serve as invaluable tools for dating ancient artifacts and fossils, unfurling the tapestry of human history.
Tips for Navigating the Isotope Labyrinth
As we delve into the captivating realm of isotopes, consider these expert tips to enrich your understanding:
Embrace the Periodic Table: The periodic table serves as an invaluable resource for exploring isotopes, providing a comprehensive overview of various elements and their isotopic compositions.
Consult Reputable Sources: Expand your isotope knowledge by delving into reputable science textbooks, peer-reviewed articles, and online databases curated by scientific organizations.
Frequently Asked Questions: Isotope Mysteries Demystified
Q: How can I determine the number of neutrons in an isotope?
A: Subtract the atomic number (number of protons) from the mass number (total number of nucleons).
Q: What practical applications stem from radioactive isotopes?
A: Radioactive isotopes fuel diverse applications, spanning nuclear power generation, medical imaging diagnostics, and cancer treatment techniques.
Q: Are all isotopes radioactive?
A: No, numerous isotopes exist in stable, non-radioactive forms, constituting the foundation of the elements we encounter in our world.
Q: How do isotopes contribute to our understanding of ancient history?
A: Radioactive isotopes, like carbon-14, enable scientists to determine the age of ancient artifacts and fossils, unraveling the mysteries of past civilizations and events.
Q: What role do isotopes play in environmental science?
A: Isotopic analysis empowers scientists to investigate environmental processes, tracciatura flows of pollutants, and monitor the impact of climate change on our planet.
What Does The Number Next To The Isotope Signify
Conclusion
The numbers that accompany element symbols in the periodic table, far from being mere notations, unveil a captivating tale of isotopic diversity, stability, and profound applications. By embracing this newfound knowledge, we gain a deeper appreciation for the intricate tapestry of chemical elements and their transformative impact on our world.
Let us embrace our fascination with isotopes, embracing the opportunity to delve further into their captivating realm. Does this topic spark your curiosity, inviting you to explore the fascinating world of isotopes and their multifaceted roles in science, technology, and our everyday lives? Share your thoughts and questions with us in the comments section below, and let us continue our collective voyage of discovery!