Have you ever pondered why water, the veritable elixir of life, exhibits a pH of 7? This seemingly mundane property holds profound implications for our understanding of the world around us and our own biological systems. Join us as we embark on an in-depth exploration into the fascinating realms of pH and pure water, unlocking the mysteries that lie within.
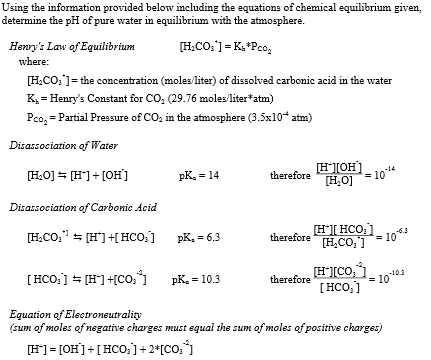
Image: www.chegg.com
pH, a measure of acidity or basicity, ranges from 0 to 14, with 7 representing neutrality. As water has a pH of 7, it falls into this neutral category. This characteristic plays a pivotal role in countless natural and man-made processes.
The Role of pH in Aquatic Ecosystems
In aquatic environments, pH directly influences the viability of aquatic organisms. Most fish, for instance, thrive in waters with a pH range of 6.5 to 8.0. Acidic waters below pH 4 can be harmful or even lethal to aquatic life, damaging gills and impairing respiratory functions.
pH also affects the availability of nutrients in water. Certain essential nutrients, such as iron and phosphate, become more or less soluble as pH changes. The optimal pH for nutrient uptake by plants and algae lies between 6.5 and 7.5.
pH in Human Biology
The human body relies on precise pH levels within its internal environment. For instance, our blood maintains a tightly regulated pH of around 7.35. A deviation from this range, known as acidosis or alkalosis, can have severe health consequences.
pH also plays a significant role in enzyme activity, the chemical reactions that power bodily functions. Enzymes have optimal pH ranges within which they exhibit maximum efficiency. Extreme pH levels can denature enzymes, disrupting their structure and impairing their ability to function.
Applications in Industry and Technology
The importance of pH extends far beyond biological systems. In industrial and technological processes, precise pH control is crucial.
In the food industry, maintaining specific pH levels ensures food safety, prevents spoilage, and enhances taste. Paper production, on the other hand, employs pH to control the viscosity and texture of paper. Additionally, pH measurement is vital in water purification systems for water treatment plants and laboratories.
Image: bluwaterlabs.com
Measuring and Adjusting pH
Accurately measuring and adjusting pH is essential for various applications. pH meters and test strips are commonly used to determine the pH of solutions or samples.
To adjust pH, acids or bases are typically added in controlled amounts. For example, in aquariums, lime or baking soda can be used to increase pH, while carbonic acid or vinegar can be used to decrease it.
Pure Water Has A Ph Of 7.
Conclusion
The significance of pure water having a pH of 7 extends far beyond a simple number. This property forms the foundation of countless natural and human-engineered systems, influencing delicate biological processes and supporting essential industrial applications.
Comprehending the intricacies of pH has empowered us to understand the intricate workings of our ecosystems and bodies. Through ongoing advancements in chemistry and technology, we continue to unravel the profound implications of pH and optimize its benefits for various spheres of life.