Life on Earth depends on the ceaseless flow of energy, in which living organisms extract chemical energy from various sources and employ it to fuel their vital processes. Respiration stands as a cornerstone of this energy-extracting machinery, enabling organisms to harness the energy stored within nutrients like glucose.
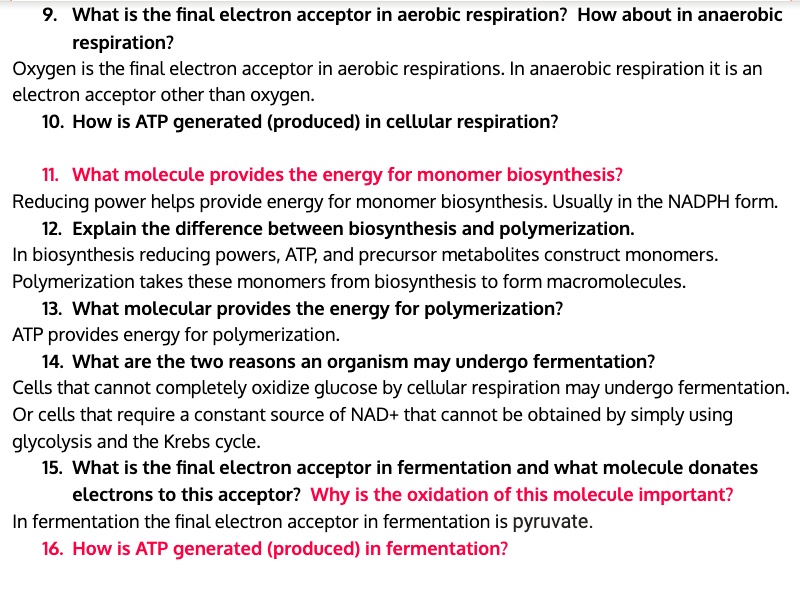
Image: www.numerade.com
While the conventional perception portrays respiration as an oxygen-dependent phenomenon, anaerobic respiration emerges as a captivating alternative, a process that liberates energy without the participation of oxygen. This fascinating metabolic pathway enables life to thrive even in oxygen-scarce environments, a testament to the adaptability of biological systems. Within this remarkable process, the final electron acceptor stands as a crucial player, receiving the electrons relinquished by glucose during the energy-generating dance.
The Anaerobic Respiratory Symphony
Anaerobic respiration, unlike its oxygen-dependent counterpart, relies on alternative electron acceptors to receive the electrons that glucose releases during its breakdown. This dance of electrons, a rhythmic transfer from one molecule to another, serves as the driving force behind anaerobic respiration.
The roster of electron acceptors for anaerobic respiration comprises a diverse cast of molecules, each lending its unique properties to the process. Nitrate, sulfate, and even carbon dioxide can ascend to the role of electron acceptor, orchestrating the intricate symphony of energy extraction.
The Nitrate Reduction Saga
In the realm of anaerobic respiration, nitrate assumes the role of a prominent electron acceptor. Nitrate reduction unravels in three distinct stages, a sequential trio of biochemical transformations that culminate in the liberation of energy.
During the first stage, nitrate embraces electrons, transforming into nitrite, akin to a graceful pirouette in the molecular ballet. Nitrite, in turn, dances forward, accepting more electrons in the second stage, morphing into nitric oxide, akin to a seamless transition of roles. The finale arrives in the third stage, where nitric oxide succumbs to further electron transfer, evolving into dinitrogen gas, the ultimate product of nitrate reduction.
The Sulfate Reduction Symphony
Sulfate, another electron acceptor of note, embarks on its own captivating respiratory saga. Similar to nitrate reduction, sulfate undergoes a stepwise transformation, shedding electrons with each graceful step.
In the initial phase, sulfate accepts electrons, shedding its former self to emerge as sulfite. Sulfite, embracing electrons once more, transforms into thiosulfate, a molecule echoing the shape of its sulfide and sulfite ancestors. The grand finale unfolds in the third stage, where thiosulfate surrenders its electrons, culminating in the formation of hydrogen sulfide, the end-product of sulfate reduction.

Image: rileyzebsmall.blogspot.com
The Carbon Dioxide Tango
Carbon dioxide, ubiquitous in its presence, also graces the stage of anaerobic respiration as an electron acceptor. This fascinating tango unfolds in two synchronized steps.
In the initial movement, carbon dioxide melds with electrons, transforming into formic acid, akin to a blending of partners in the dance of respiration. Formic acid, in turn, sways further, accepting more electrons in the second step, evolving gracefully into methane, the final product of carbon dioxide reduction.
The Diverse Cast of Anaerobic Electron Acceptors: Roles and Significance
The versatility of anaerobic respiration owes much to the diverse cast of electron acceptors it embraces. Each electron acceptor lends its unique choreography to the dance of respiration, enabling the process to flourish in a spectrum of environments.
Nitrate reduction reigns supreme in waterlogged soils, a realm where oxygen scarcity prevails. Nitrate, in this aqueous sanctuary, diligently assumes the role of electron acceptor, supporting the flourishing of denitrifying bacteria.
Sulfate reduction thrives in marine sediments, an arena where sulfate abounds. The dissimilatory sulfate-reducing bacteria, masters of this anaerobic art, orchestrate the transformation of sulfate into hydrogen sulfide, a byproduct that permeates the marine realm.
Carbon dioxide reduction finds its niche in the digestive tracts of ruminants, a haven for methanogenic archaea. These microscopic maestros wield their metabolic prowess to convert carbon dioxide into methane, a hallmark of their anaerobic endeavors.
Anaerobic Respiration: Beyond Energy Generation
The significance of anaerobic respiration extends beyond its energy-generating prowess. This intriguing process influences the cycling of elements within ecosystems. Nitrate reduction, for instance, plays a crucial role in nitrogen fixation, a vital step in the nitrogen cycle that replenishes nitrogen in the soil. Simultaneously, sulfate reduction shapes the geochemical dance of sulfur in marine ecosystems.
Moreover, anaerobic respiration holds immense promise for biotechnology and environmental applications. Microbial fuel cells, harnessing anaerobic microbial communities, offer a sustainable avenue for energy production. The ability of anaerobic microbes to degrade pollutants, such as hydrocarbons, also opens doors for environmental remediation.
What Is The Final Electron Acceptor During Anaerobic Respiration
Epilogue: Unraveling the Final Electron Acceptor
The final electron acceptor during anaerobic respiration emerges as the maestro of energy extraction, orchestrating the symphony of electron transfer that fuels microbial life in oxygen-deprived environments. Its diverse repertoire, encompassing nitrate, sulfate, and carbon dioxide, exemplifies the adaptability of life’s metabolic pathways. As we delve deeper into the intricacies of anaerobic respiration, we not only unlock the secrets of energy generation but also uncover the profound influence this process exerts on ecosystems and the potential it holds for future applications.