Ionic compounds are formed when a metal loses one or more electrons to a nonmetal. The resulting positive and negative ions are attracted to each other by electrostatic forces, forming an ionic bond.
The Structure of Ionic Compounds
Ionic compounds typically exist as crystals, where the positive and negative ions are arranged in a regular, repeating pattern. The arrangement of ions in a crystal is determined by the size and charge of the ions. Smaller ions tend to form smaller crystals, while larger ions tend to form larger crystals. The charge of the ions also affects the structure of the crystal. Ions with higher charges tend to form more tightly packed crystals.
The Properties of Ionic Compounds
Ionic compounds typically have high melting and boiling points. This is because the electrostatic forces between the ions are very strong. Ionic compounds are also good conductors of electricity when they are dissolved in water or melted. This is because the ions are free to move and carry charge.
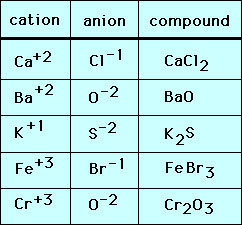
Image: cpanhd.sitehost.iu.edu
The Applications of Ionic Compounds
Ionic compounds are used in a wide variety of applications, including:
- Table salt (NaCl) is used to season food.
- Baking soda (NaHCO3) is used as a leavening agent in baking.
- Calcium chloride (CaCl2) is used to melt ice on roads.
- Potassium iodide (KI) is used to prevent iodine deficiency.
- Sodium fluoride (NaF) is used to prevent tooth decay.
The Importance of Ionic Compounds
Ionic compounds play an important role in many biological processes. For example, sodium and potassium ions are essential for the proper functioning of nerve cells. Calcium ions are essential for the proper functioning of muscles. Chlorine ions are essential for the proper functioning of the digestive system.
Ionic Compounds and Health
Some ionic compounds can be harmful to health if they are consumed in large quantities. For example, sodium chloride can raise blood pressure if it is consumed in excess. Potassium iodide can cause thyroid problems if it is consumed in excess. Calcium chloride can cause stomach upset if it is consumed in excess.

Image: sciencenotes.org
In What Form Do Most Ionic Compounds Occur
Conclusion
Ionic compounds are important chemical compounds that play a vital role in many biological processes. They are also used in a wide variety of applications, including food, medicine, and industry. Understanding the properties and applications of ionic compounds is essential for understanding the world around us.