Unlock the Enigmatic World of Electron Geometry: Delving into the Structure of SCl4
Sulfur tetrachloride, a molecule consisting of one central sulfur atom surrounded by four chlorine atoms, presents a compelling case study in electron geometry. This tetrahedral arrangement governs the shape and properties of the molecule. In this electron geometry, the valence electrons of the constituent atoms are distributed in such a way as to minimize electrostatic repulsion between them. The four electron pairs, each consisting of two electrons, occupy the corners of a tetrahedron, with the sulfur atom positioned at the center, resembling a geometric arrangement similar to that of a miniature tent.
Visualizing the Tetrahedral Framework
Envision SCl4’s structure as a three-dimensional framework, reminiscent of a regular tetrahedron, a polyhedron with four equilateral triangular faces. The sulfur atom resides at the nucleus of this tetrahedral scaffold, while the four chlorine atoms occupy its corners. Each sulfur-chlorine bond emanates from the central sulfur atom and extends towards the vertices of the tetrahedron. This tetrahedral geometry is a hallmark of molecules with four electron pairs around a central atom.
Consequences of Tetrahedral Electron Geometry
The tetrahedral electron geometry of SCl4 has profound implications for its molecular properties. First and foremost, it governs the molecule’s shape, bestowing upon it a non-linear configuration. This non-linearity renders SCl4 a non-polar molecule, meaning it lacks a permanent dipole moment. Furthermore, the tetrahedral arrangement influences the molecule’s polarity. Due to the symmetrical distribution of its electron pairs, SCl4 exhibits zero dipole moment, making it a nonpolar molecule.
Additional Factors Influencing Electron Geometry
While tetrahedral geometry is the predominant electron geometry for SCl4, factors such as the presence of lone pairs and the hybridization of atomic orbitals can introduce subtle deviations from this ideal configuration. Situations like these warrant a deeper analysis involving the hybridization of atomic orbitals, venturing beyond the scope of this introductory exploration.
Applications of SCl4: Exploring Practical Relevance
SCl4’s chemistry finds practical applications in various industrial and research domains. As a versatile chlorinating agent, SCl4 finds use in the production of dyes, pharmaceuticals, and other specialty chemicals. Researchers harness its reactivity to introduce chlorine atoms into organic molecules, paving the way for a myriad of chemical transformations.
Conclusion
The electron geometry of SCl4, a molecule with immense chemical significance, reveals a world of intricate atomic arrangements and their profound impact on molecular properties. In SCl4’s case, the tetrahedral electron geometry dictates its non-linear shape and non-polar nature. Delving into electron geometry unveils the foundations of molecular structure, bestowing upon us the ability to decipher the intricate tapestry of chemical substances that shape our world. As we continue to unravel the mysteries hidden within the world of electron geometry, we unlock the keys to understanding countless chemical phenomena. Let this exploration inspire you to embark on further journeys into the captivating realm of chemistry, uncovering the wonders that await within.
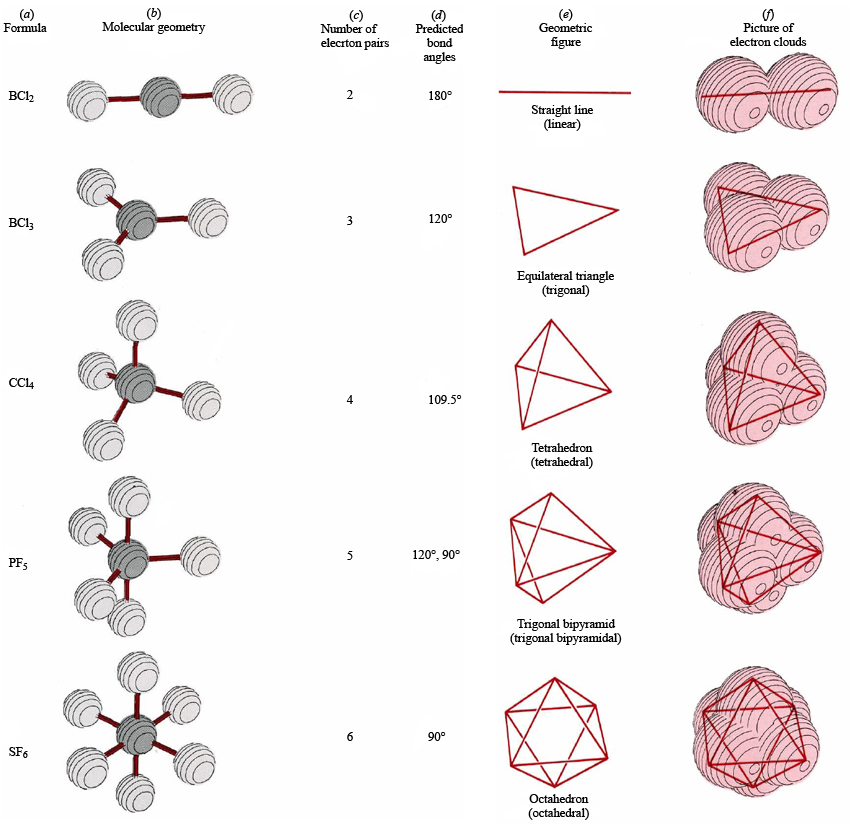
Image: socratic.com
Image: myweb.astate.edu
What Is The Electron Geometry Of Scl4